Abstract
Background: CD123 is a subunit of the interleukin 3 (IL3) receptor and is expressed on the surface of blasts in most cases of acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS). CD123 expression is often higher on leukemia cells than normal progenitors and may be enriched in residual cells surviving chemotherapy. Uniformly high CD123 is characteristic of blastic plasmacytoid dendritic cell neoplasm (BPDCN), an uncommon leukemia related to AML. Tagraxofusp (TAG, SL-401) is a recombinant protein drug consisting of IL3 fused to a truncated diphtheria toxin payload that targets CD123. Single agent TAG is approved for treatment of BPDCN. In preclinical models and in patients who received TAG, we previously found that TAG resistance in AML and BPDCN was mediated by DNA methylation and downregulation of diphthamide genes (e.g. DPH1) and subsequent resistance to diphtheria toxin (Togami, JCI 2019). TAG resistance was reversible by the hypomethylating agent azacitidine (AZA), and TAG plus AZA was more effective than either alone in xenograft models. Therefore, we performed a phase 1b trial combining TAG with AZA or with AZA and venetoclax (VEN) in patients with AML, MDS, or BPDCN.
Methods: Detection of CD123 on blasts by flow or IHC was required. Eligibility included albumin ≥3.2 g/dL, normal cardiac ejection fraction, and hospitalization in cycle 1 to mitigate risks of capillary leak syndrome (CLS). The study followed a 3+3 dose escalation, plus expansion cohorts, of 28-day cycles of TAG with fixed doses of AZA or AZA-VEN. First, we tested 5 schedules of the doublet TAG (5 or 7 μg/kg/d1-5, or 7, 9, or 12 μg/kg/d1-3) with standard doses of AZA (75 mg/m2 d1-7) in newly diagnosed AML (1L), relapsed/refractory AML (R/R), or MDS with ≥10% blasts. Next, we tested 3 doses of the triplet TAG (7, 9, or 12 μg/kg/d4-6) with AZA (75 mg/m2 d1-7) and VEN (400 mg d1-21, ramp up d1-3 in cycle 1) in 1L AML, R/R AML, or R/R BPDCN.
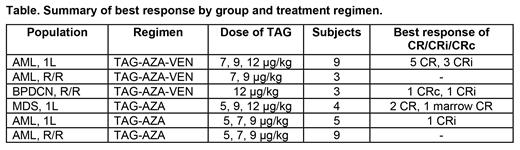
Results: 34 patients have been enrolled and 33 are evaluable (one withdrew prior to therapy). The age range is 21-86 (median 67). Median follow up is 8.4 months [95% CI, 7.1-19.1]. 18 received TAG-AZA (5 1L AML, 9 R/R AML, 4 MDS); 15 received TAG-AZA-VEN (9 1L AML, 3 R/R AML, 3 R/R BPDCN). An MTD was not reached, and the recommended phase 2 dose of TAG was determined to be 12 μg/kg/d x3d in each combination. Adverse events (AEs) were as expected for TAG or AZA+/-VEN. Grade 3+ AEs in >10% included neutropenia (30%), thrombocytopenia (27%), anemia (18%), febrile neutropenia (18%), ALT increase (12%), and bilirubin increase (12%). Eleven of 33 (33%) experienced CLS: 8 (24%) were grade 2, 2 grade 3 (6%), and 1 grade 4 (3%). One treatment-related death occurred in a 79 year-old with AML receiving TAG-AZA-VEN who experienced tumor lysis syndrome followed by CLS and multiorgan system failure during cycle 1.
Eight of 9 patients (89%) with previously untreated AML (ages 59-81, median 74; 7 of 9 ELN adverse risk) who received TAG-AZA-VEN achieved best response of complete response (CR, n=5) or complete response with incomplete count recovery (CRi, n=3), at TAG doses of 7 (n=2), 9 (n=1), or 12 μg/kg/d x3d (n=6). The 8 with CR/CRi included two AMLs with TP53 mutation and adverse karyotype, and three with secondary AML, two which had concomitant mature pDC expansion, a.k.a. "pDC-AML" (Xiao, Blood 2021). At the time of data cut off, 4 of 8 have gone to allogeneic stem cell transplantation (alloSCT) and 3 remain on therapy. Three patients with relapsed/refractory BPDCN (all had prior single agent TAG) received TAG-AZA-VEN and 2 of 3 responded (CRc=1, CRi=1), who both went to alloSCT. Four patients with previously untreated MDS and ≥10% blasts received TAG-AZA. 3 of 4 responded (all 3 with TP53 mutation) with CR (n=2) and marrow CR (n=1), and two went to alloSCT. Among 14 patients with 1L (5) or R/R (9) AML on TAG-AZA, and 3 with R/R AML on TAG-AZA-VEN, one with 1L AML (17p- and complex karyotype) achieved a CRi on TAG-AZA. Responses in all groups included patients with high and low CD123 by central flow.
Conclusions: Combining TAG with AZA or AZA-VEN is feasible, with expected TAG or AZA+/-VEN toxicities. CLS is an important consideration for TAG, requiring monitoring and early implementation of supportive care. The activity of TAG-AZA-VEN in previously untreated AML and R/R BPDCN, and of TAG-AZA in MDS are encouraging, including in high-risk AML/MDS subtypes such as TP53 mutated. These data support continued development of TAG combinations in CD123+ malignancies.
Lane: Qiagen: Consultancy, Honoraria; N-of-One: Consultancy, Honoraria; Stemline Therapeutics: Research Funding; AbbVie: Research Funding. Stein: Amgen: Consultancy, Speakers Bureau; Celgene: Speakers Bureau; Stemline: Speakers Bureau. Garcia: Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Research Funding; AstraZeneca: Research Funding; Genentech: Research Funding; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; Prelude: Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Stone: AbbVie: Consultancy; Syros: Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy; Agios: Consultancy, Research Funding; Celgene: Consultancy; Astellas: Membership on an entity's Board of Directors or advisory committees; BerGen Bio: Membership on an entity's Board of Directors or advisory committees; Boston Pharmaceuticals: Consultancy; Bristol Myers Squibb: Consultancy; Foghorn Therapeutics: Consultancy; Elevate Bio: Membership on an entity's Board of Directors or advisory committees; Onconova: Consultancy; Syndax: Membership on an entity's Board of Directors or advisory committees; Syntrix/ACI: Membership on an entity's Board of Directors or advisory committees; Arog: Consultancy, Research Funding; Gemoab: Membership on an entity's Board of Directors or advisory committees; Actinium: Membership on an entity's Board of Directors or advisory committees; Innate: Consultancy; GlaxoSmithKline: Consultancy; Janssen: Consultancy; Jazz: Consultancy; Novartis: Consultancy, Research Funding; Aprea: Consultancy; Amgen: Membership on an entity's Board of Directors or advisory committees; Macrogenics: Consultancy. Winer: Novartis: Consultancy; Takeda: Consultancy; Abbvie: Consultancy. Mughal: Oxford University Press, Informa: Other: financial benefit and/or patents ; Stemline: Current Employment, Current holder of stock options in a privately-held company. Brooks: Stemline Therapeutics: Current Employment. Konopleva: KisoJi: Research Funding; Novartis: Other: research funding pending, Patents & Royalties: intellectual property rights; Rafael Pharmaceuticals: Other: grant support, Research Funding; Sanofi: Other: grant support, Research Funding; Eli Lilly: Patents & Royalties: intellectual property rights, Research Funding; Ascentage: Other: grant support, Research Funding; Stemline Therapeutics: Research Funding; Agios: Other: grant support, Research Funding; Reata Pharmaceuticals: Current holder of stock options in a privately-held company, Patents & Royalties: intellectual property rights; Ablynx: Other: grant support, Research Funding; AstraZeneca: Other: grant support, Research Funding; Cellectis: Other: grant support; Calithera: Other: grant support, Research Funding; F. Hoffmann-La Roche: Consultancy, Honoraria, Other: grant support; Forty Seven: Other: grant support, Research Funding; Genentech: Consultancy, Honoraria, Other: grant support, Research Funding; AbbVie: Consultancy, Honoraria, Other: Grant Support, Research Funding. Pemmaraju: DAVA Oncology: Consultancy; Springer Science + Business Media: Other; Samus: Other, Research Funding; MustangBio: Consultancy, Other; Incyte: Consultancy; Clearview Healthcare Partners: Consultancy; LFB Biotechnologies: Consultancy; Stemline Therapeutics, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Abbvie Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Blueprint Medicines: Consultancy; Roche Diagnostics: Consultancy; Novartis Pharmaceuticals: Consultancy, Other: Research Support, Research Funding; Dan's House of Hope: Membership on an entity's Board of Directors or advisory committees; Plexxicon: Other, Research Funding; Celgene Corporation: Consultancy; ASCO Leukemia Advisory Panel: Membership on an entity's Board of Directors or advisory committees; HemOnc Times/Oncology Times: Membership on an entity's Board of Directors or advisory committees; Affymetrix: Consultancy, Research Funding; ASH Communications Committee: Membership on an entity's Board of Directors or advisory committees; Protagonist Therapeutics, Inc.: Consultancy; Sager Strong Foundation: Other; Daiichi Sankyo, Inc.: Other, Research Funding; Cellectis S.A. ADR: Other, Research Funding; Aptitude Health: Consultancy; CareDx, Inc.: Consultancy; Bristol-Myers Squibb Co.: Consultancy; ImmunoGen, Inc: Consultancy; Pacylex Pharmaceuticals: Consultancy.